Heavy Water - Thermophysical Properties
Thermodynamic properties of heavy water (D2O) like density, melting temperature, boiling temperature, latent heat of fusion, latent heat of evaporation, critical temperature and more.
Heavy water ( deuterium oxide , 2H2O , D2O ) is a form of water that contains a larger than normal amount of the hydrogen isotope deuterium (= heavy hydrogen = 2H = D), rather than the common hydrogen-1 isotope (1H = H = protium) that makes up most of the hydrogen in normal water.
Thermodynamic properties of heavy water - D2O:
- Boiling temperature (at 101.325 kPa): 101.40 oC = 214.52 °F
- Bulk modulus elasticity (at 25°C): 2.10×109 Pa or N/m2
- Critical density: 0.356 g/cm3 = 0.691 slug/ft3 = 3.457 lbm/gal(US)
- Critical pressure : 213.88 atm = 220.98 bar = 21.671 MPa (MN/m2) = 3143 psi (=lbf/in2)
- Critical temperature : 370.697 oC = 699.255 °F
- Ionization constant, pKw (at 25°C): 14.951
- Latent heat of evaporation (at 101.4°C): 41.521 KJ/mol = 2073.20 kJ/kg = 891.32 Btu(IT)/lb
- Latent heat of fusion: 6.132 kJ/mol = 306.2 kJ/kg = 131.64 Btu(IT)/lb
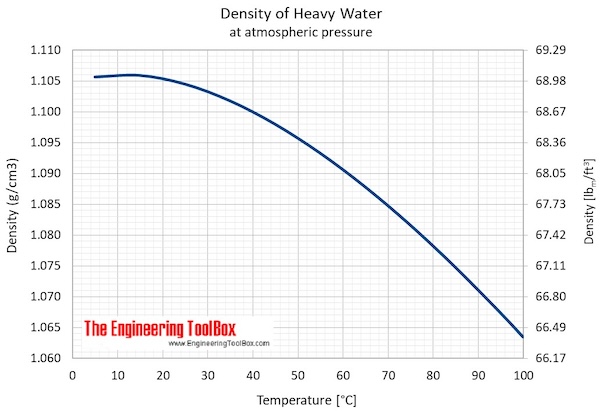
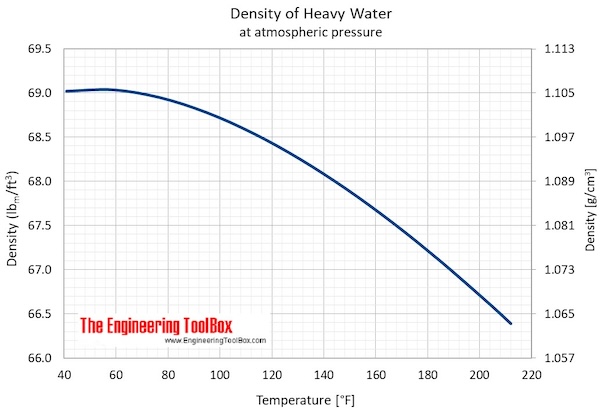
- Maximum density (at 11.23 oC): 1105.9 kg/m3 = 2.1460 slug/ft3 = 10.74048 lbm/gal(US)
- Melting temperature (at 101.325 kPa): 3.81 oC = 38.86 °F
- Molar mass: 20.02751 g/mol
- pD (~pH) (at 25°C): 7.43
- Specific heat (Cp) water (at 20°C): 4.219 kJ/kgK = 1.008 Btu(IT)/(lbm °F) or kcal/(kg K)
- Specific weight (at 11.23 oC): 10.8452 kN/m3 = 69.0391 lbf/ft3
- Surface tension (at 25°C): 71.87 dyn/cm
- Triple point pressure: 0.00652 atm = 0.00661 bar = 661 Pa = 0.0959 psi (=lbf/in2)
- Triple point temperature: 3.82 °C = 38.88 °F
- Vapor pressure (at 25°C): 20.6 mmHg = 0.027 atm = 0.028 bar = 2750 Pa = 0.398 psi
- Viscosity (at 20°C): 1.251 cP or mPa s
Ionization Constant, pKw , of normal and heavy water with varying temperature.
See also more about atmospheric pressure, and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure,
as well as Thermophysical properties of: Acetone, Acetylene, Air, Ammonia, Argon, Benzene, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethanol, Ethylene, Helium, Hydrogen, Hydrogen sulfide, Methane, Methanol, Nitrogen, Oxygen, Pentane, Propane, Toluene and Water.