Hydrogen sulfide - Thermophysical Properties
Chemical, physical and thermal properties of hydrogen sulfide, H2S, also called hydrosulfuric acid, sewer gas and stink damp. Phase diagram included.
Hydrogen sulfide, H2S, is a highly toxic and flammable, colorless gas with a characteristic odor of rotten eggs. It is used in the manufacture of chemicals, in metallurgy, and as an analytical reagent. It is heavier than air and tends to accumulate at the bottom of poorly ventilated spaces. Although very pungent at first, it quickly deadens the sense of smell.
Hydrogen sulfide occurs naturally in crude petroleum, natural gas, volcanic gases, and hot springs. It can also result from bacterial breakdown of organic matter. It is also produced by human and animal wastes.
Hydrogen sulfide is used in the manufacture of chemicals, in metallurgy, and as an analytical reagent.
The phase diagram of hydrogen sulfide is shown below the table.
Chemical, physical and thermal properties of hydrogen sulfide:
Values are given for gas phase at 25 oC (77 oF, 298 K) and 1 bara, if not other phase, temperature or pressure given.
For full table with Imperial Units - rotate the screen!
| Property | Value | Unit | Value | Unit | Value | Unit | Value | Unit | |||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Autoignition temperature | 505 | K | 232 | °C | 450 | °F | |||||
| Boiling Point | 213.6 | K | -59.55 | °C | -75.2 | °F | |||||
| Critical density | 10.2 | mol/dm3 | 348 | kg/m3 | 0.675 | slug/ft3 | 21.7 | lb/ft3 | |||
| Critical pressure | 8.97 | MPa=MN/m2 | 89.7 | bar | 88.5 | atm | 1301 | psi=lbf/in2 | |||
| Critical temperature | 373.3 | K | 100.2 | °C | 212.3 | °F | |||||
| Critical volume | 98 | cm3/mol | 0.00288 | m3/kg | 1.48 | ft3/slug | 0.0461 | ft3/lb | |||
| Density (gas) | 44.16 | mol/m3 | 1.505 | kg/m3 | 0.00292 | slug/ft3 | 0.0940 | lb/ft3 | |||
| Density (liquid) at -121.9 °F/-85.5°C | 29116 | mol/m3 | 992.3 | kg/m3 | 1.925 | slug/ft3 | 61.95 | lb/ft3 | |||
| Density (liquid) at -76 °F/-60°C | 26877 | mol/m3 | 916.0 | kg/m3 | 1.777 | slug/ft3 | 57.18 | lb/ft3 | |||
| Flammable, gas and liquid | yes | ||||||||||
| Flash point | 480 | K | 207 | °C | 405 | °F | |||||
| Gas constant, individual - R | 244.0 | J/kg K | 0.06777 | Wh/(kg K) | 1459 | (ft lbf/slug °R) | 45.34 | (ft lbf/lb °R) | |||
| Gibbs free energy of formation (gas) | -34 | kJ/mol | -998 | kJ/kg | -429 | Btu/lb | |||||
| Heat (enthalpy) of formation (gas) | -20.6 | kJ/mol | -604 | kJ/kg | -260 | Btu/lb | |||||
| Heat (enthalpy) of sublimation,at -145°F/-98°C | 25.4 | kJ/mol | 745 | kJ/kg | 320 | Btu/lb | |||||
| Heat (enthalpy) of evaporation at-100°F/-73°C | 20.00 | kJ/mol | 353.4 | kJ/kg | 151.93 | Btu/lb | |||||
| Heat capacity, Cp (gas) | 34.6 | J/mol K | 1.01 | kJ/kg K | 0.242 | Btu/lb°F or cal/g K | |||||
| Specific heat capacity, Cp (liquid) at 20 bara | 76.5 | J/mol K | 2.24 | kJ/kg K | 0.536 | Btu/lb°F or cal/g K | |||||
| Specific heat capacity, Cv (gas) | 26.0 | J/mol K | 0.76 | kJ/kg K | 0.182 | Btu/lb°F or cal/g K | |||||
| Specific heat capacity, Cv (liquid) at 20 bara | 37.0 | J/mol K | 1.09 | kJ/kg K | 0.259 | Btu/lb°F or cal/g K | |||||
| Ionization potential | 10.46 | eV | |||||||||
| Melting point | 187.66 | K | -85.5 | °C | -121.9 | °F | |||||
| Molecular Weight | 34.081 | g/mol | 0.07514 | lb/mol | |||||||
| Solubility in water, at 25°C | 4 | mg/ml | |||||||||
| Sound velocity in gas | 309 | m/s | 1014 | ft/s | 692 | mi/h | |||||
| Sound velocity in liquid at 20 bara | 836 | m/s | 2742 | ft/s | 1873 | mi/h | |||||
| Specific Gravity (gas) (relativ to air) | 1.19 | ||||||||||
| Specific Gravity (liquid) (relativ to water) | 0.92 | ||||||||||
| Specific Heat Ratio (gas) - CP/CV | 1.33 | ||||||||||
| Specific Heat Ratio (liquid) - CP/CV at 20 bara | 2.07 | ||||||||||
| Specific Volume (gas) | 0.023 | m3/mol | 0.66 | m3/kg | 342.44 | ft3/slug | 10.64 | ft3/lb | |||
| Specific Volume (liquid) at -121.9 °F/-85.5°C | 0.0000343 | m3/mol | 0.00101 | m3/kg | 0.519 | ft3/slug | 0.0161 | ft3/lb | |||
| Standard molar entropy, S° (gas) | 206 | J/mol K | 6.04 | kJ/kg K | 1.44 | Btu/lb °F | |||||
| Surface tension at 257°F/125°C | 58.1 | dynes/cm | 0.0581 | N/m | |||||||
| Thermal Conductivity | 0.018 | W/m °C | 0.010400 | Btu/hr ft °F | |||||||
| Triple point pressure | 0.0233 | MPa=MN/m2 | 0.233 | bar | 0.230 | atm | 3.38 | psi=lbf/in2 | |||
| Triple point temperature | 187.63 | K | -85.5 | °C | -121.94 | °F | |||||
| Vapor (saturation) pressure | 2.0262 | MPa=MN/m2 | 15200.0 | mm Hg | 19.9971 | atm | 293.88 | psi=lbf/in2 | |||
| Viscosity, dynamic (absolute) | 0.013 | cP | 8.6 | (lbm/ft s ×10-6) | 0.27 | (lbf s/ft2 ×10-6) | |||||
| Viscosity, kinematic | 8.505 | cSt | 91.5 | (ft2/s ×10-6) |
See more about atmospheric pressure, and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure,
as well as Thermophysical properties of: Acetone, Acetylene, Air, Ammonia, Argon, Benzene, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethanol, Ethylene, Helium, Hydrogen, Methane, Methanol, Nitrogen, Oxygen, Pentane, Propane, Toluene, Water and Heavy water, D2O.
Hydrogen sulfide is a gas at standard conditions. However, at low temperature and/or high pressures the gas becomes a liquid or a solid.
The hydrogen sulfide phase diagram shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the hydrogen sulfide boiling point with changes in pressure. It also shows the saturation pressure with changes in temperature.
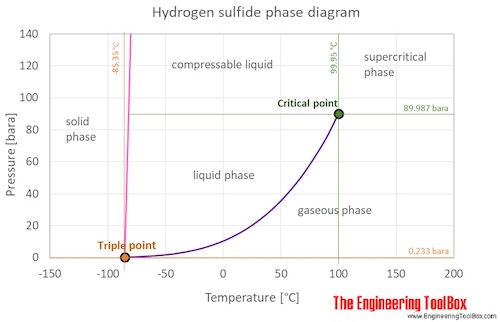
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.



