Propane - Thermophysical properties
Chemical, physical and thermal properties of propane gas - C3H8.
Chemical, physical and thermal properties of Propane - C3 H8 :
(values at 25 oC (77 oF, 298 K) and atmospheric pressure)
| Molecular Weight | 44.097 |
| Specific Gravity of gas (air = 1) | 1.52 |
| Specific Volume (ft3/lb, m3/kg) | 8.84, 0.552 |
| Density of liquid at atmospheric pressure (lb/ft3, kg/m3) | 36.2, 580 |
| Vapor pressure at 25 oC (psia, MN/m2) | 135.7, 0.936 |
| Absolute Viscosity (lbm/ft s, centipoises) | 5.38×10-6, 0.0074 |
| Sound velocity in gas (m/s) | 253 |
| Specific Heat - cp - (Btu/lb oF or cal/g oC, J/kgK) | 0.39, 1630 |
| Specific Heat Ratio - cp/cv | 1.13 |
| Gas constant - R - (ft lb/lb oR, J/kg oC) | 35.0, 188 |
| Thermal Conductivity (Btu/hr ft oF, W/moC) | 0.010, 0.017 |
| Boiling Point - saturation pressure 14.7 psia and 760 mm Hg - (oF, oC) | -44, -42.2 |
| Latent Heat of Evaporation at boiling point (Btu/lb, J/kg) | 184, 428000 |
| Freezing or Melting Point at 1 atm (oF, oC) | -309.8, -189.9 |
| Latent Heat of Fusion (Btu/lb, J/kg) | 19.1, 44400 |
| Critical Temperature (oF, oC) | 205, 96 |
| Critical Pressure (psia, MN/m2) | 618, 4.26 |
| Critical Volume (ft3/lb, m3/kg) | 0.073, 0.0045 |
| Flammable | yes |
| Heat of combustion (Btu/ft3, Btu/lb, kJ/kg) | 2450, 21660, 50340 |
See the following documents for changes in propane properties with changes in pressure and temperature :
- Density and Specific Weight
- Dynamic and Kinematic Viscosity
- Prandtl Number
- Specific Heat (Heat Capacity)
- Thermal Conductivity
- Thermal Diffusivity
- Vapor Pressure at Gas-Liquid Equilibrium
See also more about atmospheric pressure, and STP - Standard Temperature and Pressure & NTP - Normal Temperature and Pressure,
as well as Thermophysical properties of: Acetone, Acetylene, Air, Ammonia, Argon, Benzene, Butane, Carbon dioxide, Carbon monoxide, Ethane, Ethanol, Ethylene, Helium, Hydrogen, Hydrogen sulfide, Methane, Methanol, Nitrogen, Oxygen, Pentane, Toluene, Water and Heavy water, D2O . Propane is a gas at standard conditions. However, at low temperature and/or high pressures the gas becomes a liquid (or a solid at very cold conditions).
The phase diagram for propane shows the phase behavior with changes in temperature and pressure. The curve between the critical point and the triple point shows the propane boiling point with changes in pressure. It also shows the saturation pressure with changes in temperature.
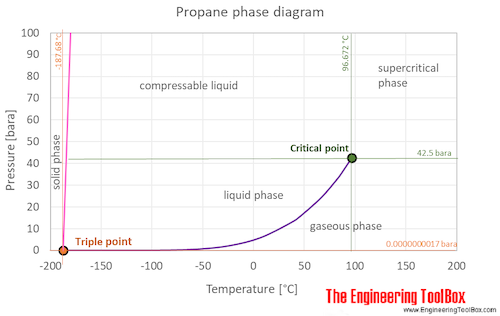
At the critical point there is no change of state when pressure is increased or if heat is added.
The triple point of a substance is the temperature and pressure at which the three phases (gas, liquid, and solid) of that substance coexist in thermodynamic equilibrium.



