Air - Drying Force
The drying force of air depends on the air moisture holding capacity and the water surface to air evaporation capacity.
Air is used in product drying processes for heat and vapor transport.
It is common to transfer heat required to evaporate water from a product from heating coils in a dryer to the drying products by the continuously circulation of air. Evaporated water vapor from the product is removed by replacing some of the circulating air with fresh make-up air with lower specific moisture content.
The drying force of the air is the difference between vapor pressure in the air and saturation pressure at the same temperature. The drying force can be expressed as:
DF = pws - pw (1)
where
DF = Drying Force (mbar, Pa, psi)
pw = vapor pressure (mbar, Pa, psi)
pws = saturation vapor pressure at the actual dry bulb temperature (mbar, Pa, psi)
Note! The drying force is not a force as known from the mechanics (Newton). It express the most import variable for the vapor carrying capacity of humid air.
The table below indicates vapor saturation pressure related to temperature.
| Temperature | Saturation Vapor Pressure (10-3bar) | |
|---|---|---|
| (oC) | (oF) | |
| -18 | 0 | 1.5 |
| -15 | 5 | 1.9 |
| -12 | 10 | 2.4 |
| -9 | 15 | 3.0 |
| -7 | 20 | 3.7 |
| -4 | 25 | 4.6 |
| -1 | 30 | 5.6 |
| 2 | 35 | 6.9 |
| 4 | 40 | 8.4 |
| 7 | 45 | 10.3 |
| 10 | 50 | 12.3 |
| 13 | 55 | 14.8 |
| 16 | 60 | 17.7 |
| 18 | 65 | 21.0 |
| 21 | 70 | 25.0 |
| 24 | 75 | 29.6 |
| 27 | 80 | 35.0 |
| 29 | 85 | 41.0 |
| 32 | 90 | 48.1 |
| 35 | 95 | 56.2 |
| 38 | 100 | 65.6 |
| 41 | 105 | 76.2 |
| 43 | 110 | 87.8 |
| 46 | 115 | 101.4 |
| 49 | 120 | 116.8 |
| 52 | 125 | 134.2 |
- 10-3 bar = 1 millibar
Example - The Drying Force of Air
Air is heated from 21 oC and 50% relative humidity (A) to 38 oC (B).
With the saturation pressure from the table above and the expression for relative humidity the vapor pressure in (A) can be expressed as:
pw = (25 mbar) (50%) / (100%)
= 12.5 (mbar)
The drying force in A can be calculated as:
DFA = (25 mbar) - (12.5 mbar)
= 12.5 (mbar)
Heating the air from A to B don't change the moisture content. The vapor pressure remains constant but the saturation pressure increases. The relative humidity decreases to 19% - the Mollier diagram.
The vapor pressure in B can be calculated as:
pw = (65.6 mbar) (19%) / (100%)
= 12.5 (mbar)
The drying force in B can be calculated as:
DFB = (65.6 mbar) - (12.5 mbar)
= 53.1 mbar
Comparing A and B the "Drying Force" has increased from 12.5 mbar to 53.1 mbar. This has the double effect
- moisture transport capacity of the air is increased
- evaporation capacity from water surfaces in the air is increased
Note! - air temperature has major influence on drying capacity
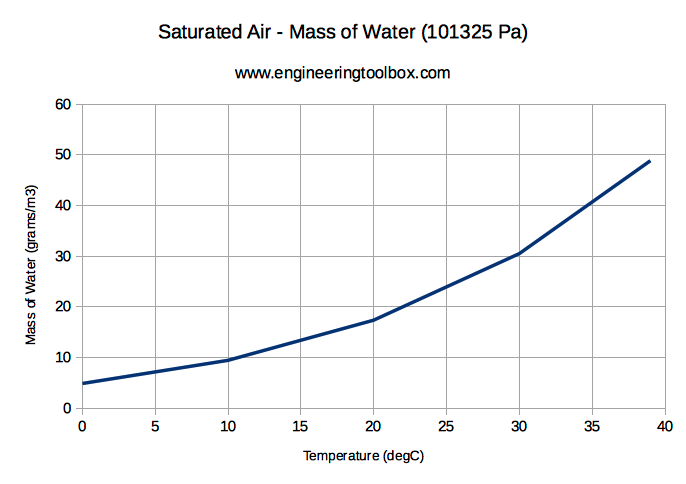
Saturated Air and Mass of Water