Moist Air - Water Vapor and Saturation Pressure
Saturation pressure of water vapor in moist air vs. temperature.
Water vapor is almost always present in the surrounding air.
Note! - the equations below is for pure water vapor - not moist air.
Saturation Pressure of Water Vapor
The maximum saturation pressure of the water vapor in moist air varies with the temperature of the air vapor mixture and can be expressed as:
pws = e(77.3450 + 0.0057 T - 7235 / T)/ T8.2 (1)
where
pws = water vapor saturation pressure (Pa)
e = the constant 2.718.......
T = dry bulb temperature of the moist air (K)
Density of Water Vapor
The density of water vapor can be expressed as:
ρw = 0.0022 pw / T (2)
where
p w = partial pressure water vapor (Pa, N/m2)
ρ w = density water vapor (kg/m3)
T = absolute dry bulb temperature (K)
Saturation pressure and density of water vapor for common temperatures
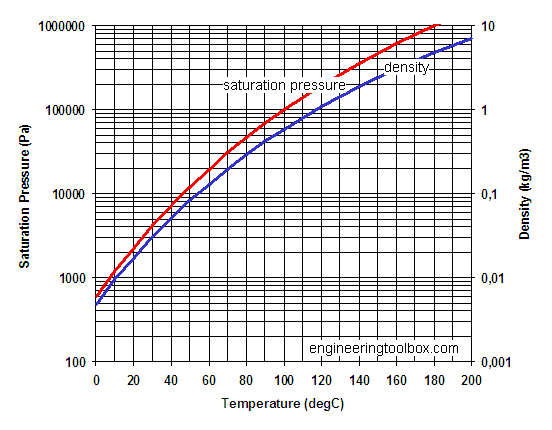
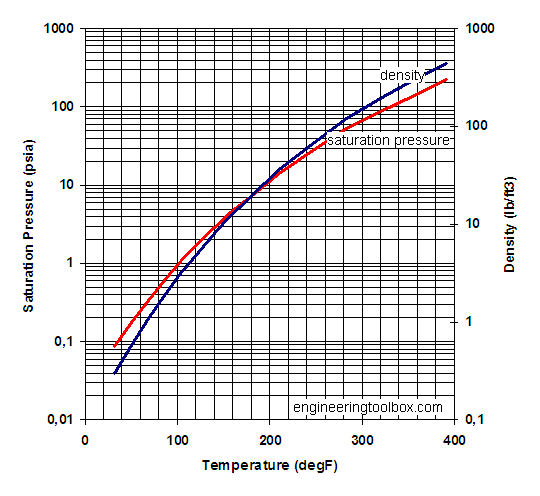
| Temperature | Saturation Pressure | Density | |||||
|---|---|---|---|---|---|---|---|
| (oC) | (oF) | (Pa) | (mmHg) | (psia) | (inHg) | (kg/m3) | 10-3 (lb/ft3) |
| 0 | 32 | 603 | 4.6 | 0.09 | 0.18 | 0.005 | 0.30 |
| 10 | 50 | 1212 | 9.2 | 0.18 | 0.36 | 0.009 | 0.59 |
| 20 | 68 | 2310 | 17.4 | 0.33 | 0.68 | 0.017 | 1.08 |
| 30 | 86 | 4195 | 31.7 | 0.61 | 1.24 | 0.030 | 1.90 |
| 40 | 104 | 7297 | 55.1 | 1.06 | 2.15 | 0.051 | 3.20 |
| 50 | 122 | 12210 | 92.2 | 1.8 | 3.60 | 0.083 | 5.19 |
| 60 | 140 | 19724 | 149 | 2.9 | 5.82 | 0.13 | 8.13 |
| 70 | 158 | 30866 | 233 | 4.5 | 9.11 | 0.20 | 12.3 |
| 80 | 176 | 46925 | 354 | 6.8 | 13.8 | 0.29 | 18.2 |
| 90 | 194 | 69485 | 525 | 10.1 | 20.5 | 0.42 | 26.3 |
| 100 | 212 | 100446 | 758 | 14.6 | 29.6 | 0.59 | 36.9 |
| 120 | 248 | 196849 | 1486 | 28.6 | 58.1 | 1.10 | 68.7 |
| 140 | 284 | 358137 | 2704 | 51.9 | 105.7 | 1.91 | 119 |
| 160 | 320 | 611728 | 4619 | 88.7 | 180.5 | 3.11 | 194 |
| 180 | 356 | 990022 | 7475 | 144 | 292.1 | 4.80 | 300 |
| 200 | 392 | 1529627 | 11549 | 222 | 451.2 | 7.11 | 444 |
- 1 lb/ft3 = 16.018 kg/m3
- 1 kg/m3 = 0.0624 lb/ft3
Example - Saturation Pressure Water Vapor
The Saturation pressure of water vapor in moist air at dry bulb temperature 25 oC can be calculated:
First, convertion from °C to K:
( 25 °C) + 273 = 298 (K)
Then Eq. (1) is used:
pws = e(77.3450 + 0.0057 (298 K) - 7235 / (298 K))/ 298 (K)8.2
= 3130 (Pa)



