Moist Air - Density vs. Water Content and Temperature
Density of the mix of dry air and water vapor - moist humid air.
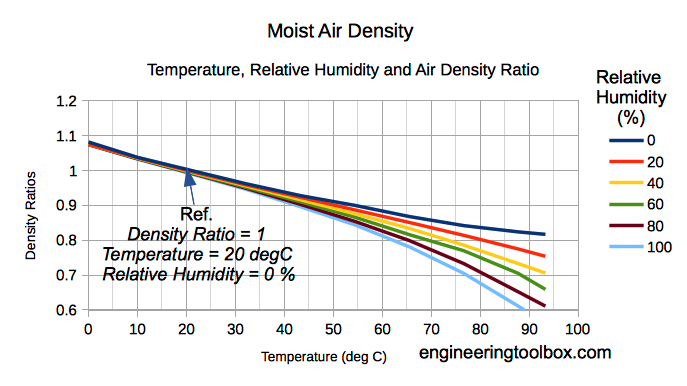
The density of humid air varies with water content and temperature. When the temperature increases a higher molecular motion results in expansion of volume and a decrease of density.
The density of a gas, dry air, water vapor - or a mixture of dry air and water vapor like moist or humid air - can be calculated with the Ideal Gas Law.
Density of Dry Air
The density of dry air can be calculated
ρa = pa / Ra T
= 0.0035 pa / T (1)
where
ρ a = density dry air (kg/m3)
pa = partial pressure of dry air (Pa, N/m2)
Ra = 286.9 - individual gas constant of dry air (J/kg K)
T = absolute dry bulb temperature (K)
Density of Water Vapor
The density of the water vapor can be calculated
ρw = pw / Rw T
= 0.0022 pw / T (2)
where
p w = partial pressure of water vapor (Pa, N/m2)
ρ w = density of water vapor (kg/m3)
Rw = 461.5 - individual gas constant water vapor (J/kg K)
T = absolute dry bulb temperature (K)
Density of Moist Air - an Air Vapor Mixture
The amount of water vapor in air influences the density. Water vapor is relatively light compared to diatomic Oxygen and diatomic Nitrogen - the dominant components in air.
When vapor content increases in moist air the amount of Oxygen and Nitrogen are decreased per unit volume and the density of the mix decreases since the mass is decreasing.
Based on specific volume of moist air the moist air density can be calculated
ρ = 1 / v
= (p / Ra T) (1 + x) / (1 + x Rw / Ra ) (3)
where
v = specific volume of moist air per mass unit of dry air and water vapor (m3/kg)
x = mw / ma = specific humidity or humidity ratio (kgh2o /kgdry_air )
mw = mass of water vapor (kg)
ma = mass of dry air (kg)
p = pressure in the moist air (Pa)
The density of dry air with the same total pressure p as in the mixture (dry air + vapor partial pressure) - can be calculated as a reference as
ρref = p / Ra T (4)
where
ρref = reference density of dry air with the same pressure as in the air vapor mixture (kg/m3)
The density of moist air related to dry air can be expressed by combining (4) and (3) as
ρ = ρref (1 + x) / (1 + x Rw / Ra ) (5)
The gas constant ratio between water vapor and air is
Rw / Ra = (461.5 J/kg K) / (286.9 J/kg K)
= 1.609
and inserting the ratio in (5) the density of moist air related to dry air can be expressed as
ρ = ρref (1 + x) / (1 + 1.609 x) (6)
Note! As we can see from (6) - increased moisture content reduces the density of moist air - dry air is more dense than moist air.