Elements of the Periodic System
The elements of the periodic system with names, symbols, atomic numbers and weights, melting and boiling points, density, electronegativity and electron affinity, and electron configuration.
Figures and tables showing the elements from the periodic table - their name, symbol, atomic number, average atomic weight, state at 273 K, melting and boiling point, density at standard temperature and pressure, electronegativity, electron affinity, electron configuration and first ionisation energy.
Average atomic mass is the same number as molar mass, however, the unit for molar mass is g/mol.
For elements without stable isotops, the mass number for the isotop with the longest half-life is given in parenthesis.
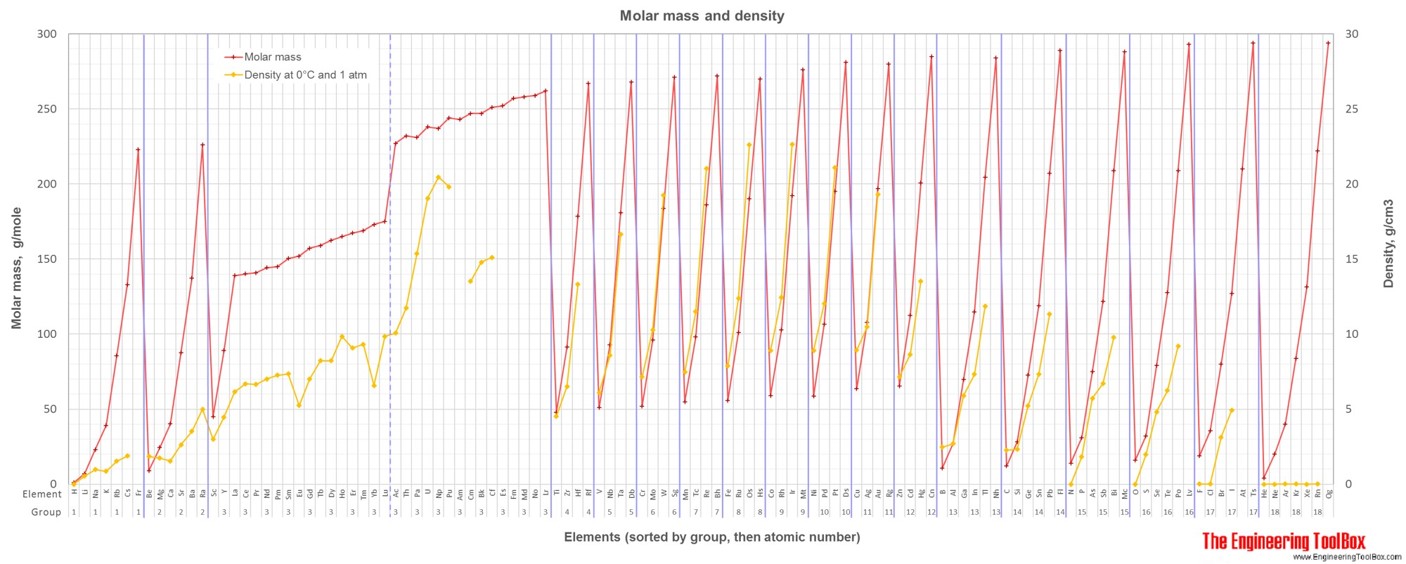
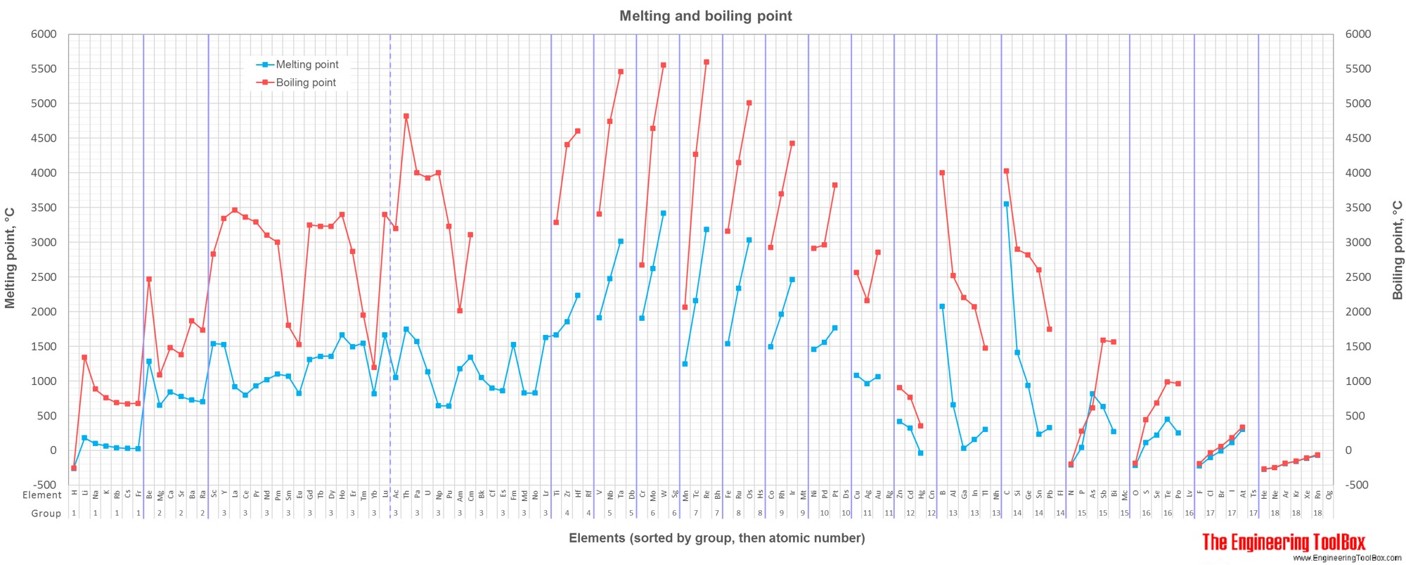
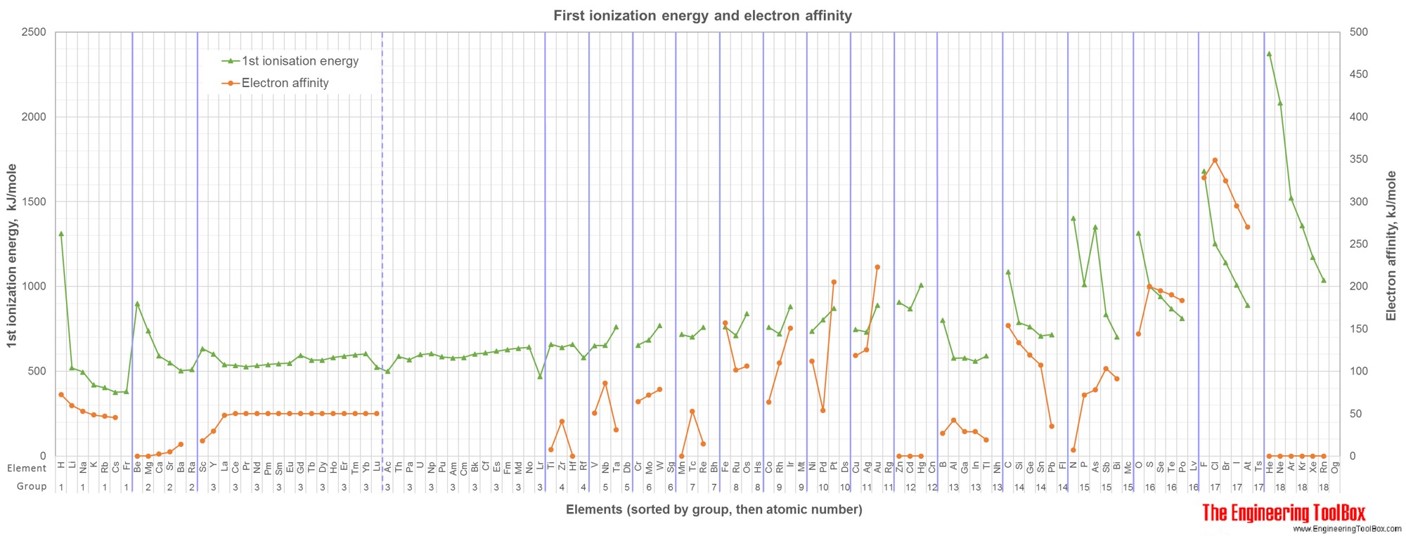
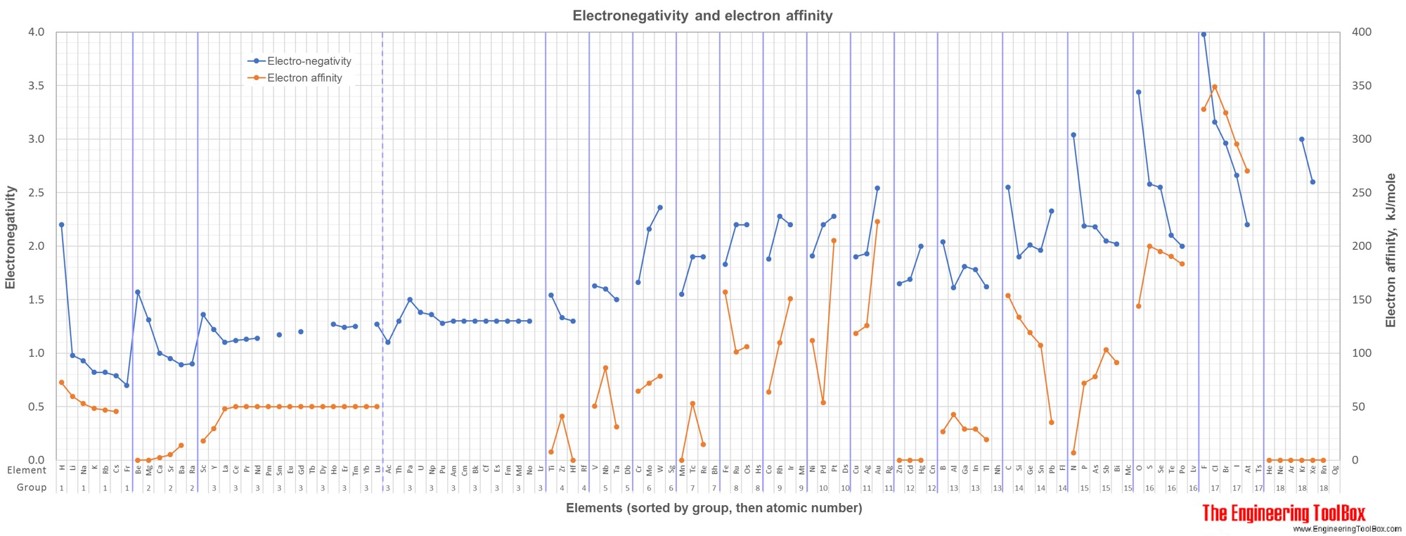
For full table with boiling points, density, electronegativity - rotate the screen!
| Element Name | Element Symbol | Atomic Number | Avarage atomic Mass1) (u) | State at 273 K | Melting point2) (°K/°C/°F) | Boiling point3 (°K/°C/°F) | Density at stp4) (g/cm3) | Electro-negativity5) | Electron affinity6) (kJ/mole) | Electron configuration per shell7) (#) | 1st ionisation energy8 (kJ/mol) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Actinium | Ac | 89 | (227) | Solid | 1323/1059/1922 | 3473/3200/5792 | 10.07 | 1.1 | 2,8,18,32,18,9,2 | 499 | |
| Aluminum | Al | 13 | 26.981538 | Solid | 933/660/1221 | 2792/2519/4566 | 2.70 | 1.61 | 42.5 | 2,8,3 | 577.5 |
| Americium | Am | 95 | (243) | Solid | 1449/1176/214 | 2284/2011/3652 | 1.3 | 2,8,18,32,25,8,2 | 578 | ||
| Antimony (Stibium) | Sb | 51 | 121.760 | Solid | 904/631/1167 | 1860/1587/2888 | 6.697 | 2.05 | 103.2 | 2,8,18,18,5 | 834 |
| Argon | Ar | 18 | 39.948 | Gas | 84/ -189/ -309 | 87/-186/-303 | 0.001784 | 0 | 2,8,8 | 1520.6 | |
| Arsenic | As | 33 | 74.92160 | Solid | 1090/817/1502 | 887/614/1137 | 5.727 | 2.18 | 78 | 2,8,18,5 | 1350.8 |
| Astatine | At | 85 | (210) | Solid | 575/302/575 | 610/337/638 | 2.2 | 270.1 | 2,8,18,32,18,7 | 890 | |
| Barium | Ba | 56 | 137.327 | Solid | 1000/727/1340 | 2143/1870/3398 | 3.510 | 0.89 | 13.95 | 2,8,18,18,8,2 | 502.9 |
| Berkelium | Bk | 97 | (247) | Solid | 1323/1050/1922 | 14.780 | 1.3 | 2,8,18,32,27,8,2 | 601 | ||
| Beryllium | Be | 4 | 9.012182 | Solid | 1560/1287/2348 | 2743/2470/4478 | 1.848 | 1.57 | 0 | 2,2 | 899.5 |
| Bismuth | Bi | 83 | 208.98038 | Solid | 544/271/520 | 1837/1564/2847 | 9.780 | 2.02 | 91.2 | 2,8,18,32,18,5 | 703 |
| Boron | B | 5 | 10.811 | Solid | 2348/2075/3767 | 4273/4000/7232 | 2.460 | 2.04 | 26.7 | 2,3 | 800.6 |
| Bromine | Br | 35 | 79.904 | Liquid | 266/-7/19 | 332/59/138 | 3.120 | 2.96 | 324.6 | 2,8,18,7 | 1139.9 |
| Cadmium | Cd | 48 | 112.411 | Solid | 594/21/610 | 1040/767/1412 | 8.650 | 1.69 | 0 | 2,8,18,18,2 | 867.8 |
| Calcium | Ca | 20 | 40.078 | Solid | 1115/842/1547 | 1757/1484/2703 | 1.550 | 1.0 | 2.37 | 2,8,8,2 | 589.8 |
| Californium | Cf | 98 | (251) | Solid | 1173/900/1652 | 15.10 | 1.3 | 2,8,18,32,28,8,2 | 608 | ||
| Carbon | C | 6 | 12.0107 | Solid | 3823/3550/6422 | 4300/4027/7280 | 2.260 | 2.55 | 153.9 | 2,4 | 1086.5 |
| Cerium | Ce | 58 | 140.116 | Solid | 1071/798/1468 | 3633/3360/6080 | 6.689 | 1.12 | 50 | 2,8,18,19,9,2 | 534.4 |
| Cesium | Cs | 55 | 132.90545 | Solid | 302/28/83 | 944/671/1240 | 1.879 | 0.79 | 45.5 | 2,8,18,18,8,1 | 375.7 |
| Chlorine | Cl | 17 | 35.453 | Gas | 172/-102/-151 | 239/-34/-29 | 0.003214 | 3.16 | 349 | 2,8,7 | 1251.2 |
| Chromium | Cr | 24 | 51.9961 | Solid | 2180/1907/3464 | 2944/2671/4840 | 7.140 | 1.66 | 64.3 | 2,8,13,1 | 652.9 |
| Cobalt | Co | 27 | 58.933200 | Solid | 1768/1495/2723 | 3200/2927/5300 | 8.900 | 1.88 | 63.7 | 2,8,15,2 | 760.4 |
| Copper | Cu | 29 | 63.546 | Solid | 1358/1085/1984 | 2835/2562/4643 | 8.920 | 1.90 | 118.4 | 2,8,18,1 | 745.5 |
| Curium | Cm | 96 | (247) | Solid | 1618/1345/2453 | 3383/3110/5630 | 13.510 | 1.3 | 2,8,18,32,25,9,2 | 581 | |
| Dysprosium | Dy | 66 | 162.50 | Solid | 1629/1356/2473 | 3503/3230/5846 | 8.219 | 50 | 2,8,18,27,8,2 | 565.8 | |
| Einsteinium | Es | 99 | (252) | Solid | 1133/860/1580 | 1.3 | 2,8,18,32,29,8,2 | 619 | |||
| Erbium | Er | 68 | 167.259 | Solid | 1770/1497/2726 | 3141/2868/5194 | 9.066 | 1.24 | 50 | 2,8,18,30,8,2 | 589.3 |
| Europium | Eu | 63 | 151.964 | Solid | 1095/822/1511 | 1800/1527/2780 | 5.244 | 50 | 2,8,18,25,8,2 | 547.1 | |
| Fermium | Fm | 100 | (257) | Solid | 1800/1527/2780 | 1.3 | 2,8,18,32,30,8,2 | 627 | |||
| Fluorine | F | 9 | 18.9984032 | Gas | 53/-220/-364 | 85/-188/-307 | 0.001696 | 3.98 | 328 | 2,7 | 1681.0 |
| Francium | Fr | 87 | (223) | Solid | 300/27/80 | 950/677/1250 | 0.7 | 2,8,18,32,18,8,1 | 380 | ||
| Gadolinium | Gd | 64 | 157.25 | Solid | 1586/1313/2395 | 3523/3250/5882 | 7.01 | 1.2 | 50 | 2,8,18,25,9,2 | 593.4 |
| Gallium | Ga | 31 | 69.723 | Solid | 303/30/86 |
2477/2204/3999 |
5.904 | 1.81 | 28.9 | 2,8,18,3 | 578.8 |
| Germanium | Ge | 32 | 72.64 | Solid | 1211/938/1721 | 3093/2820/5108 | 5.23 | 2.01 | 119 | 2,8,18,4 | 762 |
| Gold (Aurum) | Au | 79 | 196.96655 | Solid | 1337/1064/1948 | 3129/2856/5173 | 19.300 | 2.54 | 222.8 | 2,8,18,32,18,1 | 890.1 |
| Hafnium | Hf | 72 | 178.49 | Solid | 2506/2233/4051 | 4876/4603/8317 | 13.310 | 1.3 | 0 | 2,8,18,32,10,2 | 658.5 |
| Helium | He | 2 | 4.002602 | Gas | 0/-273/-460 | 4/-269/-452 | 0.0001785 | 0 | 2 | 2372.3 | |
| Holmium | Ho | 67 | 164.93032 | Solid | 1936/1663/3025 | 3675/3402/6155 | 9.841 | 1.27 | 50 | 2,8,18,29,8,2 | 581.0 |
| Hydrogen | H | 1 | 1.00794 | Gas | 14/-259/-434 | 20/-253/-423 | 0.0000899 | 2.20 | 72.5 | 1 | 1312.0 |
| Indium | In | 49 | 114.818 | Solid | 430/157/314 | 2345/2072/3761 | 7.310 | 1.78 | 28.9 | 2,8,18,18,3 | 558.3 |
| Iodine | I | 53 | 126.90447 | Solid | 387/114/237 | 457/184/364 | 4.940 | 2.66 | 295.2 | 2,8,18,18,7 | 1008.4 |
| Iridium | Ir | 77 | 192.217 | Solid | 2739/2466/4471 | 4701/4428/8002 | 22.650 | 2.20 | 151 | 2,8,18,32,15,2 | 880 |
| Iron (Ferrum) | Fe | 26 | 55.845 | Solid | 1811/1538/2800 |
3434/2861/5182 |
7.874 | 1.83 | 157 | 2,8,14,2 | 762.5 |
| Krypton | Kr | 36 | 83.798 | Gas | 116/-157/-251 | 120/-153/-244 | 0.00375 | 3.0 | 0 | 2,8,18,8 | 1358.8 |
| Lanthanum | La | 57 | 138.9055 | Solid | 1193/920/1688 | 3737/3464/6267 | 6.146 | 1.10 | 48 | 2,8,18,18,9,2 | 538.1 |
| Lawrencium | Lr | 103 | (262) | Solid | 1900/1628/2960 | 2,8,18,32,32,8,3 | 470 | ||||
| Lead (Plumbum) | Pb | 82 | 207.2 | Solid | 601/327/621 | 2022/1749/3180 | 11.340 | 2.33 | 35.1 | 2,8,18,32,18,4 | 715.6 |
| Lithium | Li | 3 | 6.941 | Solid | 454/181/357 | 1615/1342/2447 | 0.535 | 0.98 | 59.6 | 2,1 | 520.2 |
| Lutetium | Lu | 71 | 174.967 | Solid | 1936/1663/3025 | 3675/3402/6155 | 9.841 | 1.27 | 50 | 2,8,18,32,9,2 | 523.5 |
| Magnesium | Mg | 12 | 24.3050 | Solid | 923/650/1202 | 1363/1090/1994 | 1.738 | 1.31 | 0 | 2,8,2 | 737.7 |
| Manganese | Mn | 25 | 54.9380 | Solid | 1519/1246/2275 | 2334/2061/3742 | 7.470 | 1.55 | 0 | 2,8,13,2 | 717.3 |
| Mendelevium | Md | 101 | (258) | Solid | 1100/827/1520 | 1.3 | 2,8,18,32,31,8,2 | 635 | |||
| Mercury (Hydrargyrum) | Hg | 80 | 200.59 | Liquid | 234/-39/-38 | 630/357/674 | 13.534 | 2.0 | 0 | 2,8,18,32,18,2 | 1007.1 |
| Molybdenum | Mo | 42 | 95.94 | Solid | 2896/2623/4753 | 4912/4639/8382 | 10.280 | 2.16 | 71.9 | 2,8,18,13,1 | 684.3 |
| Neodymium | Nd | 60 | 144.24 | Solid | 1294/1021/1870 | 3373/3100/5612 | 7.010 | 1.14 | 50 | 2,8,18,22,8,2 | 533.1 |
| Neon | Ne | 10 | 20.1797 | Gas | 25/-249/-415 | 27/-246/-411 | 0.0009 | 0 | 2,8 | 2080.7 | |
| Neptunium | Np | 93 | (237) | Solid | 917/644/1191 | 4273/4000/7232 | 20.450 | 1.36 | 2,8,18,32,22,9,2 | 604.5 | |
| Nickel | Ni | 28 | 58.6934 | Solid | 1728/1455/2651 | 3186/2913/5275 | 8.908 | 1.91 | 112 | 2.8.16.2 | 737.1 |
| Niobium (Columbium) | Nb | 41 | 92.90638 | Solid | 2750/2477/4490 | 5017/4744/8571 | 8.570 | 1.6 | 86.1 | 2,8,18,12,1 | 652.1 |
| Nitrogen | N | 7 | 14.0067 | Gas | 63/-210/-346 | 77/-196/-320 | 0.001251 | 3.04 | 7 | 2,5 | 1402.3 |
| Nobelium | No | 102 | (259) | Solid | 1100/827/1520 | 1.3 | 2,8,18,32,32,8,2 | 642 | |||
| Osmium | Os | 76 | 190.23 | Solid | 3306/303/5491 | 5285/5012/9053 | 22.610 | 2.2 | 106.1 | 2,8,18,32,14,2 | 840 |
| Oxygen | O | 8 | 15.9994 | Gas | 55/-218/-361 | 90/-183/-297 | 0.001429 | 3.44 | 144 | 2,6 | 1313.9 |
| Palladium | Pd | 46 | 106.42 | Solid | 1828/1555/2831 | 3236/2963/5365 | 12.023 | 2.20 | 53.7 | 2,8,18,18 | 804.4 |
| Phosphorus | P | 15 | 30.973761 | Solid | 317/44/111 | 554/280/537 | 1.823 | 2.19 | 72 | 2,8,5 | 1011.8 |
| Platinum | Pt | 78 | 195.078 | Solid | 2041/1768/3215 |
4098/3825/6917 |
21.090 | 2.28 | 205.3 | 2,8,18,32,17,1 | 870 |
| Plutonium | Pu | 94 | (244) | Solid | 913/640/1184 | 3503/3230/5846 | 19.816 | 1.28 | 2,8,18,32,24,8,2 | 584.7 | |
| Polonium | Po | 84 | (209) | Solid | 527/254/489 | 1235/962/1763 | 9.196 | 2.0 | 183.3 | 2,8,18,32,18,6 | 812.1 |
| Potassium (Kalium) | K | 19 | 39.0983 | Solid | 337/63/146 | 1032/759/1398 | 0.856 | 0.82 | 48.4 | 2,8,8,1 | 418.8 |
| Praseodymium | Pr | 59 | 140.90765 | Solid | 1204/931/1708 | 3563/3290/5954 | 6.640 | 1.13 | 50 | 2,8,18,21,8,2 | 527 |
| Promethium | Pm | 61 | (145) | Solid | 1373/1100/2012 | 3273/3000/5432 | 7.264 | 50 | 2,8,18,23,8,2 | 540 | |
| Protactinium | Pa | 91 | 231.03588 | Solid | 1845/1572/2861 | 4273/4000/232 | 15.370 | 1.5 | 2,8,18,32,20,9,2 | 568 | |
| Radium | Ra | 88 | (226) | Solid | 973/700/1292 | 2010/1737/3158 | 5.0 | 0.9 | 2,8,18,32,18,8,2 | 509.3 | |
| Radon | Rn | 86 | (222) | Gas | 202/-71/-96 | 211/-62/-79 | 0.00973 | 0 | 2,8,18,32,18,8 | 1037 | |
| Rhenium | Re | 75 | 186.207 |
Solid |
3459/3186/5767 | 5869/5596/10105 | 21.020 | 1.9 | 14.5 | 2,8,18,32,13,2 | 760 |
| Rhodium | Rh | 45 | 102.90550 | Solid | 2237/1964/3567 | 3968/3695/6683 | 12.450 | 2.28 | 109.7 | 2,8,18,16,1 | 719.7 |
| Rubidium | Rb | 37 | 85.4678 | Solid | 312/39/103 | 961/688/1270 | 1.532 | 0.82 | 46.9 | 2,8,18,8,1 | 403.0 |
| Ruthenium | Ru | 44 | 101.07 | Solid | 2607/2334/4233 | 4423/4150/7502 | 12.370 | 2.2 | 101.3 | 2,8,18,15,1 | 710.2 |
| Samarium | Sm | 62 | 150.36 | Solid | 1345/1072/1961 | 2076/1803/3277 | 7.353 | 1.17 | 50 | 2,8,18,24,8,2 | 544.5 |
| Scandium | Sc | 21 | 44.955910 | Solid | 1814/1541/2806 | 3103/2830/5126 | 2.985 | 1.36 | 18.1 | 2,8,9,2 | 633.1 |
| Selenium | Se | 34 | 78.96 | Solid | 494/221/430 | 958/685/1265 | 4.819 | 2.55 | 195 | 2,8,18,6 | 941.0 |
| Silicon | Si | 14 | 28.0855 | Solid | 1687/1414/2577 | 3173/2900/5252 | 2.330 | 1.90 | 133.6 | 2,8,4 | 786.5 |
| Silver (Argentum) | Ag | 47 | 107.8682 | Solid | 1235/962/1763 | 2435/2162/3923 | 10.490 | 1.93 | 125.6 | 2,8,18,18,1 | 731.0 |
| Sodium (Natrium) | Na | 11 | 22.989770 | Solid | 371/98/208 | 1156/883/1621 | 0.968 | 0.93 | 52.8 | 2,8,1 | 495.8 |
| Strontium | Sr | 38 | 87.62 | Solid | 1050/777/1430 | 1655/1382/2519 | 2.630 | 0.95 | 5.03 | 2,8,18,8,2 | 549.5 |
| Sulfur | S | 16 | 32.065 | Solid | 388/115/239 | 718/445/832 | 1.960 | 2.58 | 200 | 2,8,6 | 999.6 |
| Tantalum | Ta | 73 | 180.9479 | Solid | 3290/3017/5462 | 5731/5458/9856 | 16.650 | 1.5 | 31 | 2,8,18,32,11,2 | 761 |
| Technetium | Tc | 43 | (98) | Solid | 2430/2457/3914 | 4538/4265/7709 | 11.500 | 1.9 | 53 | 2,8,18,13,2 | 702 |
| Tellurium | Te | 52 | 127.60 | Solid | 723/450/841 | 1261/988/1810 | 6.240 | 2.1 | 190.2 | 2,8,18,18,6 | 869.3 |
| Terbium | Tb | 65 | 158.92534 | Solid | 1629/1356/2473 | 3503/3230/5846 | 8.219 | 50 | 2,8,18,27,8,2 | 565.8 | |
| Thallium | Tl | 81 | 204.3833 | Solid | 577/304/579 | 1746/1473/2683 | 11.850 | 1.62 | 19.2 | 2,8,18,32,18,3 | 589.4 |
| Thorium | Th | 90 | 232.0381 | Solid | 2023/1750/3182 | 5093/4820/8708 | 11.724 | 1.3 | 2,8,18,32,18,10,2 | 587 | |
| Thulium | Tm | 69 | 168.93421 | Solid | 1818/1545/2813 |
2223/1950/3542 |
9.321 | 1.25 | 50 | 2,8,18,31,8,2 | 596.7 |
| Tin (Stannum) | Sn | 50 | 118.710 | Solid | 505/232/449 | 2875/2602/4715 | 7.310 | 1.96 | 107.3 | 2,8,18,18,4 | 708.6 |
| Titanium | Ti | 22 | 47.867 | Solid | 1941/1668/3034 | 3560/3287/5948 | 4.507 | 1.54 | 7.6 | 2,8,10,2 | 658.8 |
| Tungsten (Wolfram) | W | 74 | 183.84 | Solid | 3695/3422/6191 | 5828/5555/10031 | 19.250 | 2.36 | 78.6 | 2,8,18,32,12,2 | 770 |
| Uranium | U | 92 | 238.02891 | Solid | 1408/1135/2075 | 4200/3927/7100 | 19.050 | 1.38 | 2,8,18,32,21,9,2 | 597.6 | |
| Vanadium | V | 23 | 50.9415 | Solid | 2183/1910/3170 | 3680/3407/6164 | 6.110 | 1.63 | 50.6 | 2,8,11,2 | 650.9 |
| Xenon | Xe | 54 | 131.293 | Gas | 161/-112/-169 |
165/-108/-162 |
0,0059 | 2.6 | 0 | 2,8,18,18,8 | 1170.4 |
| Ytterbium | Yb | 70 | 173.04 | Solid | 1092/819/1506 | 1469/1196/2185 | 6.570 | 50 | 2,8,18,32,8,2 | 603.4 | |
| Yttrium | Y | 39 | 88.90585 | Solid | 1799/1526/2779 | 3618/3345/6053 | 4.472 | 1.22 | 29.6 | 2,8,18,9,2 | 600 |
| Zinc | Zn | 30 | 65.409 | Solid | 693/420/787 | 1180/907/1664 | 7.140 | 1.65 | 0 | 2,8,18,2 | 906.4 |
| Zirconium | Zr | 40 | 91.224 | Solid | 2128/1855/3371 | 4682/4409/7968 | 6.511 | 1.33 | 41.1 | 2,8,18,10,2 | 640.1 |
1) Standard average atomic weights (IUPAC 1997) for the isotops naturally present in the element.
- u = atomic mass unit, 1 u = 1.66×10-27 kg
2) Melting point is the temperature at which a substance changes from solid to liquid state. Temperature converter
3) Boiling point is the temperature at which a substance changes from liquid to gas state.
4) Density (mass/volume) at standard temperature and pressure, given as gram/cm3. Standard temperature is equal to 0°C or 32°F and standard pressure is equal to 1 atm, 101.3kPa or 760 mmHg (torr)
5) Electronegativity given by the Pauling scale. Electronegativity is a measure of the tendency of an atom to attract a bonding pair of electrons. The Pauling scale is the most commonly used. Fluorine (the most electronegative element) is assigned a value of 4.0, and values range down to caesium and francium which are the least electronegative at 0.7.
6) Electron affinity is most commonly defined as the energy released (Einitial+Efinal) when an additional electron is attached to a neutral atom or molecule(in gaseous phase) to form a negative ion. Another, equivalent definition is the energy required to detach an electron from the singly charged negative ion (energy for the process X-(g) -> X(g) + e-). It could also be described as a neutral atom's likelihood of gaining an electron. The values vary from 0 to 349 kJ/mole
7) Electron configuration per shell lists the number of electrons in each shell of electrons, starting from the inner shell.
8) 1st ionisation energy is the energy required to remove the most loosely held electron from one mole of gaseous atoms to produce 1 mole of gaseous ions each with a charge of 1+: X(g) --> X+(g) + e- It could also be described as a neutral atom's likelihood of giving away an electron. Ionisation energies are measured in kJ mol-1 (kilojoules per mole). They vary from 380 up to 2372 kJ/mole.



