Solubility of Air in Water
The amount of air that can be dissolved in water decreases with temperature and increases with pressure.
The amount of air that can be dissolved in water increases with pressure and decreases with temperature.
Deaeration
When fresh water is heated up air bubbles start to form. The water can obviously not hold the dissolved air with increased temperature. At 100 oC (212 oF) water starts to boil - the bubbles are formed by evaporated water or steam. If the water is cooled down and then again reheated, bubbles will not appear until the water starts to boil. The water is deaerated.
Solubility Ratio
Solubility of air in water can be expressed as a solubility ratio:
Sa = ma / mw (1)
where
Sa = solubility ratio
ma = mass of air (lbm, kg)
mw = mass of water (lbm, kg)
Henry's Law
Solubility of air in water follows Henry's Law - "the amount of air dissolved in a fluid is proportional to the pressure in the system" - and can be expressed as:
c = pg kH (2)
where
c = solubility of dissolved gas
kH = proportionality constant depending on the nature of the gas and the solvent
pg = partial pressure of gas (Pa, psi)
The solubility of oxygen in water is higher than the solubility of nitrogen. Air dissolved in water contains approximately 35.6% oxygen compared to 21% in air.
Solubility of Air in Water
Solubility of air in water - expressed as ratio of absorbed air volume to water volume:
Temperature (oF) | Solubility (vair/vwater) | |||||
|---|---|---|---|---|---|---|
| Gauge Pressure (psig) | ||||||
| 0 | 20 | 40 | 60 | 80 | 100 | |
| 40 | 0.0258 | 0.0613 | 0.0967 | 0.1321 | 0.1676 | 0.2030 |
| 50 | 0.0223 | 0.0529 | 0.0836 | 0.1143 | 0.1449 | 0.1756 |
| 60 | 0.0197 | 0.0469 | 0.0742 | 0.1014 | 0.1296 | 0.1559 |
| 70 | 0.0177 | 0.0423 | 0.0669 | 0.0916 | 0.1162 | 0.1408 |
| 80 | 0.0161 | 0.0387 | 0.0614 | 0.0840 | 0.1067 | 0.1293 |
| 90 | 0.0147 | 0.0358 | 0.0569 | 0.0780 | 0.0990 | 0.1201 |
| 100 | 0.0136 | 0.0334 | 0.0532 | 0.0730 | 0.0928 | 0.1126 |
| 110 | 0.0126 | 0.0314 | 0.0501 | 0.0689 | 0.0877 | 0.1065 |
| 120 | 0.0117 | 0.0296 | 0.0475 | 0.0654 | 0.0833 | 0.1012 |
| 130 | 0.0107 | 0.0280 | 0.0452 | 0.0624 | 0.0796 | 0.0968 |
| 140 | 0.0098 | 0.0265 | 0.0432 | 0.0598 | 0.0765 | 0.0931 |
| 150 | 0.0089 | 0.0251 | 0.0413 | 0.0574 | 0.0736 | 0.0898 |
| 160 | 0.0079 | 0.0237 | 0.0395 | 0.0553 | 0.0711 | 0.0869 |
| 170 | 0.0068 | 0.0223 | 0.0378 | 0.0534 | 0.0689 | 0.0844 |
| 180 | 0.0055 | 0.0208 | 0.0361 | 0.0514 | 0.0667 | 0.0820 |
| 190 | 0.0041 | 0.0192 | 0.0344 | 0.0496 | 0.0647 | 0.0799 |
| 200 | 0.0024 | 0.0175 | 0.0326 | 0.0477 | 0.0628 | 0.0779 |
| 210 | 0.0004 | 0.0155 | 0.0306 | 0.0457 | 0.0607 | 0.0758 |
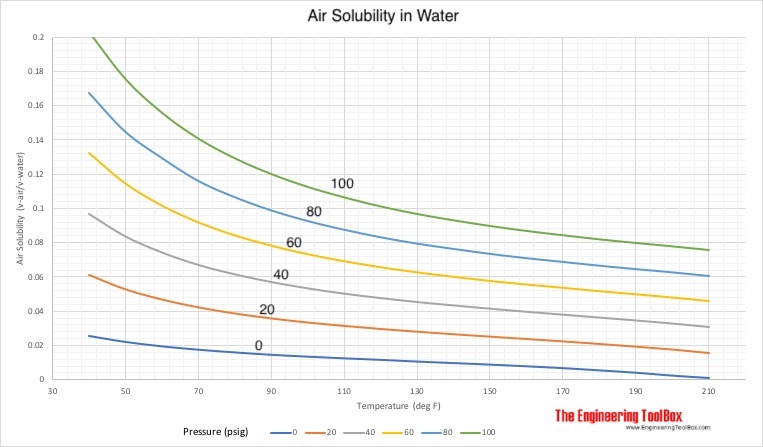
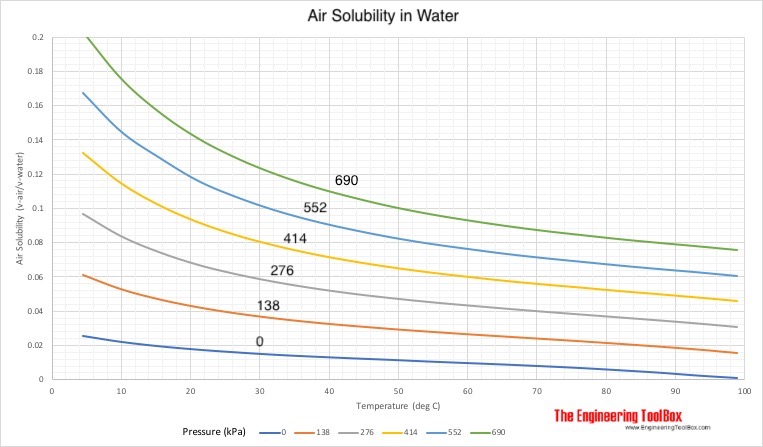
Example - Air Dissolved in Water
The amount of air dissolved in water - mass of air to volume/mass of water - can be calculated with Henry's law.
Henry Law's Constants at a system temperature of 25 oC (77 oF)
- Oxygen - O2 : 756.7 atm/(mol/litre)
- Nitrogen - N2 : 1600 atm/(mol/litre)
Molar Weights
- Oxygen - O2 : 31.9988 g/mol
- Nitrogen - N2 : 28.0134 g/mol
Partial fraction in Air
- Oxygen - O2 : ~ 0.21
- Nitrogen - N2 : ~ 0.79
Oxygen dissolved in the Water at atmospheric pressure can be calculated as:
co = (1 atm) 0.21 / (756.7 atm/(mol/litre)) (31.9988 g/mol)
=0.0089 g/litre
~ 0.0089 g/kg
Nitrogen dissolved in the Water at atmospheric pressure can be calculated as:
cn = (1 atm) 0.79 / (1600 atm/(mol/litre)) (28.0134 g/mol)
=0.0138 g/litre
~ 0.0138 g/kg
Since air mainly consists of Nitrogen and Oxygen - the air dissolved in the water can be calculated as:
ca =(0.0089 g/litre) + (0.0138 g/litre)
= 0.0227 g/litre
~ 0.023 g/kg
Calculating the air dissolved in water for some other pressures at temperature 25 oC (77 oF) can be summarized to:
| Pressure, abs (atm) | 1 | 2 | 3 | 4 | 5 | 6 |
| Dissolved Air in Water (25 oC) (g/kg) | 0.023 | 0.045 | 0.068 | 0.091 | 0.114 | 0.136 |
Dissolved Oxygen in Fresh Water
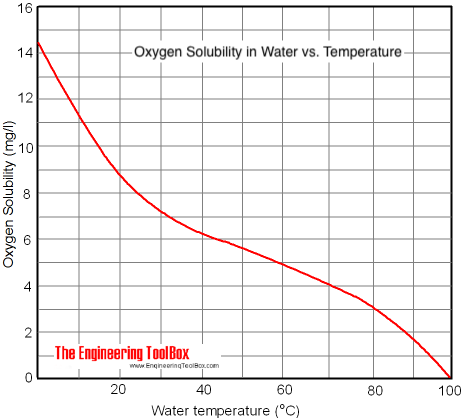
Deaeration
For maximum deaeration the water should be heated up to 212 oF (100 oC) at atmospheric pressure. This is common in steam systems where fresh water is supplied to the system through a heated deaeration tower on the top of the condensate receiver tank.
It is also common to install deaeration devices on the hot sides of heat exchangers in heating distribution systems to force the dissolved air out of the system.
Note! Since the maximum deaeration is limited by the minimum static pressure and maximum temperature in the system - the best deaeration result is achieved in positions at the hottest and highest levels of the systems - and/or at the suction side of pumps.



