Metal Alloys - Melting Points
Alloys and their melting points.
An alloy is a mixture of metals or a mixture of a metal and another element. Melting points of some mixtures of metals are indicated in the diagrams below:
Lead - Pb
Melting points of Pb - Lead - in mixtures with
- Sn - Tin
- Bi - Bismuth
- Te - Tellurium
- Ag - Silver
- Na - Sodium - Natrium
- Cu - Copper
- Sb - Antimony

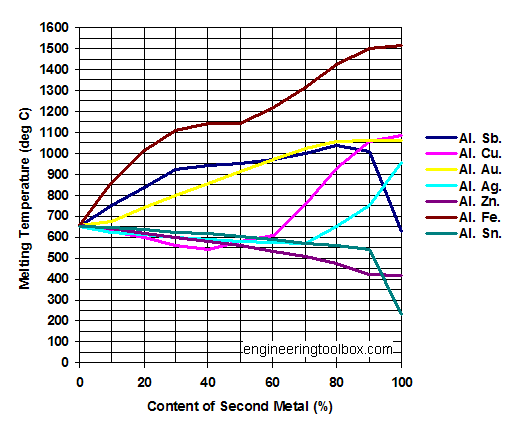
Aluminum - Al
Melting points of Al - Aluminum - in mixtures with
- Sb - Antimony
- Cu - Copper
- Au - Gold
- Ag - Silver
- Zn - Zinc
- Fe - Iron
- Sn - Tin
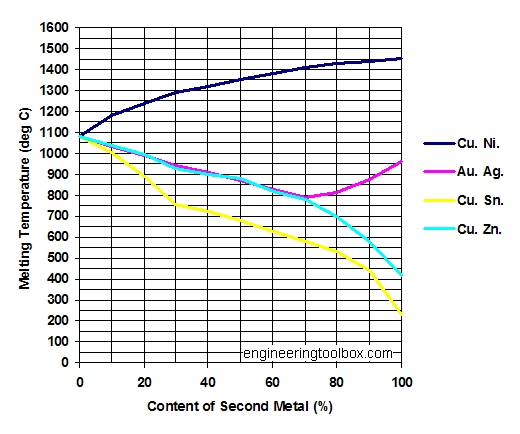
Copper - Cu
Melting points of Cu - Copper - in mixtures with
- Ni - Nickel
- Ag - Silver
- Sn - Tin
- Zn - Zinc
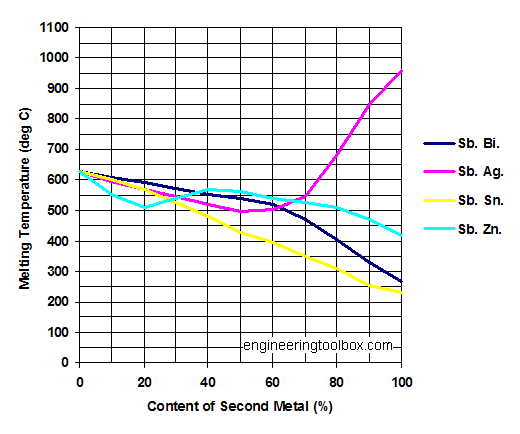
Antimony - Sb
Melting points of Sb - Antimony - in mixtures with
- Bi - Bismuth
- Ag - Silver
- Sn - Tin
- Zn - Zinc