Capillarity
Capillarity - or capillary action - is the ability of a narrow tube to draw a liquid upwards against the force of gravity.
Capillarity - or capillary action - is the ability of a narrow tube to draw a liquid upwards against the force of gravity.
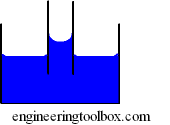
The height of liquid in a tube due to capillarity can be calculated
h = 2 σ cos(θ) / (ρ g r) (1)
where
h = height of liquid (ft, m)
σ = surface tension (lb/ft, N/m)
θ = contact angle (θ = 0 for clean tube)
ρ = density of liquid (lb/ft3, kg/m3)
g = acceleration of gravity (32.174 ft/s2, 9.81 m/s2)
r = radius of tube (ft, m)
Surface Tension
Surface tension is typically measured in dynes /cm or N/m .
| Liquid | Surface Tension - σ - | |
|---|---|---|
| N/m | dynes/cm | |
| Ethyl Alcohol | 0.0223 | 22.3 |
| Mercury | 0.465 | 465 |
| Water 20 oC | 0.0728 | 72.75 |
| Water 100 oC | 0.0599 |
58.9 |
Capillarity, like surface tension, decreases with increasing temperature. The temperature variation, however, is small and insignificant in most problems.
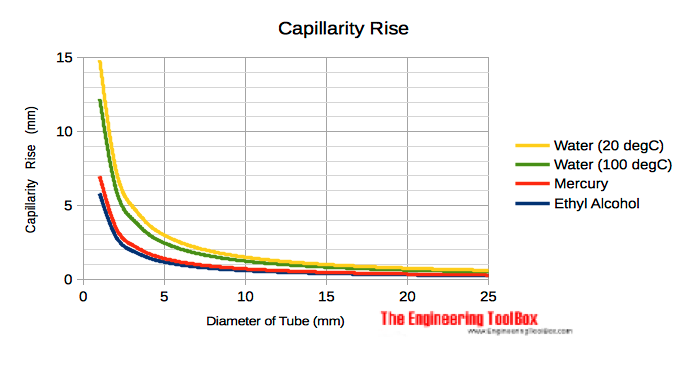
Example - Water Capillarity Rise in Tube
The capillarity rise in a clean tube (θ = 0) with diameter 2 mm and water temperature 20 oC with density 1000 kg/m3 can be calculated as
h = 2 (0.0728 N/m) cos(0 degrees) / ((1000 kg/m3) (9.81 m/s2) (2×10-3 m))
= 0.0074 m
= 7.4 mm
Capillarity Rise in Tubes
Capillarity rise in clean circular glass tubes for distilled water, fresh water and mercury at temperature 20 oC (68 oF) :